Why do cancers start?

.
Numerous other promoter oncogenes have been discovered so far which, when activated, promote development, abnormal growth and spread of many cancers. Targeting the function of these oncogenes with antibodies or drugs such as tyrosine kinase inhibitors has resulted in several successful anti-cancer biological therapies, including:
HER-2 (breast) – trastuzumab (Herceptin)
- VEGF (colon, breast, kidney) – bevacizumab (Avastin)
- B-RAF (melanoma) – vemurafenib (Zelboraf) and dabrafenib (Tafinlar)
- C-KIT (GIST tumours) – imatinib (Gleevec) and nilotinib (Tasigna)
- EGFR (Lung) – erlotinib
- EGFR (Colon) – cetuximab (Erbitux)
Many other oncogenes, such as a mutated form of RAS (pancreas, prostate) and Cyclin D1 (head and neck, oesophagus), have been shown to cause cancer and the race is on to develop drugs which target the abnormal metabolic processes caused by their overexpression.
Essentially we are born with the tendency to get cancer – it is part of us! DNA damage leading to amplification or mutated versions of our own genes could result in four scenarios:
- Immediate death of the cell
- Damage which the cell repairs and subsequent normal growth
- No repaired but the damage doesn’t affect growth regulation
- Not repaired and the cells continue to grow dysfunctionally
Many other pathways are required before these genetically dysfunctional cells develop into full-blown cancer. It has been estimated that standing in strong sun for a few hours will generate over three thousand genetic dysfunctional cells which could go on to form cancer, but these are dealt with efficiently by immune killer cells.
,
Why do some people get cancer and others don’t?
The World Cancer Research Fund and Cancer Research UK (CRUK) estimate that up to 50% of cancers are likely to be related to lifestyle as opposed to genetic susceptibility or just chance spontaneous formation. Furthermore, the World Health Organization estimate that the fraction of cancers attributable to carcinogen exposure is between 10-20%.
Some people are fortunate to be born with their cancer genes locked in tightly by stable suppressor genes, resulting in a very robust DNA profile. Others have DNA which can be easily damaged. or worse, have been born with defective genes or repair mechanisms which make cancer almost inevitable. Most people, however, are somewhere in between these two extremes, but whatever risk group you are in, a healthy lifestyle can reduce the odds of cancer forming, delay the age of diagnosis or result in less aggressive types developing.

Even if an individual has a defective genetic syndrome which carries an increased cancer risk, the severity of the syndrome can change from one person to another. This is called penetrance and is often affected by other genes which affect similar biochemical pathways. Lifestyle choices also have a considerable influence on how genes are expressed. Other important factors include the strength of an individual’s immune system and exposure to carcinogens.
1. Genetic cancer susceptibility
Unfortunately, some people have genetic defects in their genes which increase the risk of cancer, with these susceptible genes often passed onto future generations. The few inherited cancers that doctors can test for at the moment include those linked with breast, ovarian, bowel and womb cancers. Consider testing if you have:
- Two or more first-degree relative (brother, sister, child or parent) with breast or ovarian cancer, especially if they are under 50 years
- One first-degree relative with prostate cancer < 60 years
- Two or more second-degree relative, (grandfather, grandson, uncle and nephew diagnosed) with the same cancer
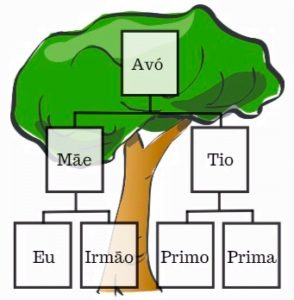
Types and mechanisms of genetic cancer susceptibility
There are several classes of germline mutations in genes (genes you were born with) that may be involved in cancer development and promotion. Fortunately, they are rare and, even if present, they can alter in their ability to cause cancer (Penetrance). Only a minority of cancers are caused by germline mutations, with the vast majority (90%) linked to acquired mutations and environmental factors. The main susceptibility genes include:
- Tumour suppressor genes
- Oncogenes
- Genes involved in DNA repair and cell cycle control
- Genes involved in stimulating the angiogenic pathways
Tumour suppressor genes
Conditions linked to TSG include:
- Inherited retinoblastoma syndrome – eye
- Familial adenomatous polyposis (FAP) – polyps and bowel
- Gorlin Syndrome – skin cancers
- Cowden syndromes – skin, muscle, brain
- Neurofibromatosis types 1 and 2 – skin, brain
These genes keep the bad genes in check, so their normal function is important. However, these genes can either be damaged directly or rearranged from the abnormal genes they are suppressing via damage to the DNA. This lack of control initiates the cancer sequence.
Oncogenes
Inherited mutations in oncogenes are rarer, and syndromes are often associated with growth defects, Noonan’s syndrome or multiple adenomas. RAS oncogenes seem to be particularly important as, when activated, they have been shown to promote cancer growth. There does not seem to be any syndromes which enhance RAS expression, but it is overexpressed in many cancers. A prospective trial involving men with low-risk prostate cancer found that RAS oncogenes were down-regulated after exercise and polyphenol-rich foods.
DNA repair genes
Some genes control pathways which help mend DNA mutations and maintain genetic stability, protecting us from cancer. DNA repair defects are a common cause of inherited cancer susceptibility, and examples include:
- Ataxia telangiectasia
- Fanconi anaemia
- Xeroderma pigmentosum
- Hereditary non-polyposis colon cancer (Lynch syndrome)
- Breast cancer susceptibility – BRCA1 and BRCA2
Alterations in genes which are involved in the vascular endothelial growth factor (VEGF) pathway, can lead to abnormal stimulation of blood vessel growth (angiogenesis). Individuals with these mutations either have von Hippel Lindau disease or something similar and are at high risk of rare cancers such as:
- cerebellar haemangioblastomas,
- renal cell cancers
- phaeochromocytomas
People with these mutations require careful monitoring at least annually, but fortunately, treatment of these tumours with anti-angiogenic agents is increasingly effective.
.
2. Medical interventions to reduce cancer risks
These can both increase and decrease the risk of cancer. Radiotherapy to the abdomen of young men with testicular seminoma has been shown to increase the risk of bowel and bladder cancer, while women with Hodgkin’s Lymphoma have an increased risk of breast cancer after mantle radiotherapy to their chest. On the other hand, several medical interventions can reduce the risk of cancer.
Screening for early or precancerous cancers – general public
Breast mammography
- Faecal blood analysis
- PSA screen
Targeted exploratory procedures for at-risk populations
- Breast MRI in BRCA carriers or those previously irradiated
- Regular colonoscopy – previous cancers, polyps, or genetic risks
- Regular cystoscopy – previous bladder polyps
Prophylactic surgery
- Double mastectomy – BRCA mutation carriers
- Removal of ovaries – BRCA mutation carriers
- Removal of large bowel – genetic risk or ulcerative colitis sufferers
Chemoprevention
- Vaccination of HPV to prevent cervical cancer
- Tamoxifen for women with a higher risk of breast cancer
- Aspirin for people with a history of bowel polyps
As our understanding of what genetic and biochemical factors cause cancer develops, it is very likely that more chemopreventative strategies will be available, especially in the area of vaccinations against cancers.
 3. Immunity
3. Immunity
The interaction between the immune system and cancer is complex and extremely important. An intact, functioning immune system is vital in killing thousand of early cancer cells which form every day. For established cancers, a robust immune system ensures the effectiveness of biological therapies that recruit killer cells and other natural weapons. On the other hand, a chronically dysfunctional immune-inflammatory response can increase the risk of cancer and enhance its progression.
4. Carcinogens and acquired genetic susceptibility

5. Epigenetics

In terms of cancer, if epigenetic processes increase expression of a tumour suppressor gene and decrease expression of a tumour promotor gene, this would lead to a reduced risk of cancer or slower cancer progression. On the other hand, if adverse factors such as chronic inflammation and continued carcinogen exposure influence the epigenetic processes to switch off suppressor genes and switch on promotor genes, cancer is more likely to develop and progress.

– Exercise >>
– Polyphenol and phytochemical intake >>
– Processed sugar intake >>
– Body composition >>
– Mineral and vitamin status >>
– Healthy and unhealthy fat balance >>
– Psychological status >>
 HER-2 (breast) – trastuzumab (Herceptin)
HER-2 (breast) – trastuzumab (Herceptin)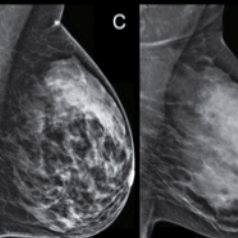 Breast mammography
Breast mammography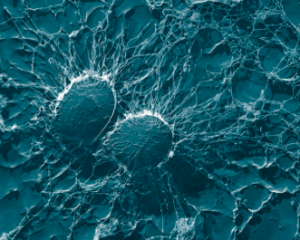 3. Immunity
3. Immunity