Cellular energy (respiration)
Our bodies primarily use sugars broken down from carbohydrates for energy, but if sugar levels drop in the bloodstream, cells use fats and, if needed, proteins. The biochemical pathways responsible for energy are ultimately responsible for the formation of a chemical called adenosine triphosphate (ATP), derived from adenosine diphosphate ADP. These pathways are also involved in the formation of a chemical called reduced nicotinamide adenine dinucleotide (NADH). When either ATP or NADH are then broken down, energy is released – they are essentially the fuel for cells.
– ATP = ADP + energy
– NADH = NAD+ + energy
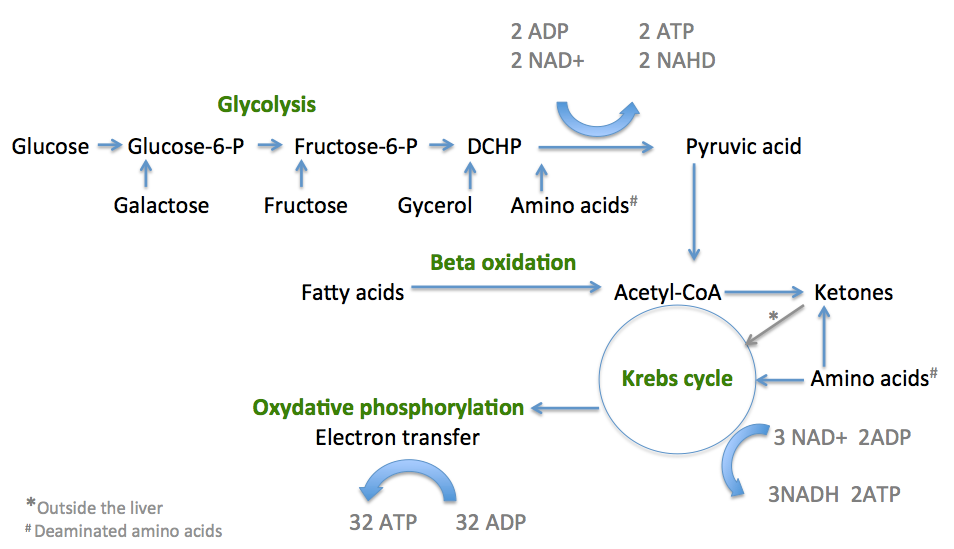
The energy used by human cells requires the metabolism of 100 – 150 moles of ATP daily, which is around 50 to 75 kg. A human will, therefore, typically use up his or her body weight of ATP over the course of the day. The process of producing these energy-storing molecules is called cellular respiration. The three phases of cellular respiration are:
- Glycolysis – producing 2 ATP and 2 NADH
- The critic acid (TCA) cycle or Kreb’s cycle – producing 2 ATP + 4NADH
- Oxidative phosphorylation (OXOPHOS) – producing 32 ATP
Diagram summarising the pathways of energy production
.
Glycolysis

Citric acid cycle
Oxidative phosphorylation
Oxidative phosphorylation also occurs in the mitochondria and requires a great deal of oxygen being transported to the cells from the lungs. Oxidative phosphorylation is made up of two closely connected components – the electron transport chain and chemiosmosis. In the electron transport chain, NADH moves from outside to inside the mitochondria, where it donates an electron to the electron transport chain. The electron transport chain consists of a group of proteins (and some lipids) that work together to pass electrons “down the line”, forming an electrochemical gradient producing 32 ATP’s providing energy for many cellular functions.
Utilising fats, proteins and ketones to produce energy
The monosaccharides glucose, fructose and galactose are the body’s preferred primary source, but in times of fasting, it can easily use fats instead. If starvation has set in, proteins can be used, although this essentially amounts to the body eating its own muscles. This is commonly seen in severely ill patients who have had trouble eating or absorbing food for some time – a state called cachexia. Ketones can be used by cells outside the liver and some cells can even use lactic acid.
Fats as cellular energy sources

Proteins as cellular energy sources

Ketones as energy sources
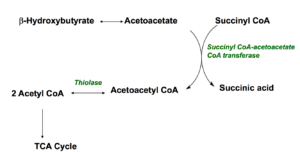
Ketones are produced when the body burns fats or proteins to produce energy molecules. They are also produced when there is not enough insulin to help your body use sugar for energy, such as during an uncontrolled type 1-diabetic crisis. As mentioned above, fatty acids are changed via a series of reactions called beta-oxidation into ketones, then acetyl CoA molecules, which enter the Krebs cycle to produce energy molecules. The three main ketones are; Acetone, B-hydroxybutyrate and acetoacetate.
Acetone is volatile and excreted in the breath, while B-hydroxybutyrate is converted to acetoacetate. To understand why this mechanism is a survival advantage, it is important to note that liver cells lack the enzyme (Succinyl CoA transferase) which concerts ketones to acetyl CoA. Instead, ketones are released into the bloodstream, enabling them to feed other tissues such as the brain, muscles and heart. In these tissues (which have the enzyme) the ketones are converted back to acetyl CoA, which then enters the Krebs cycle to produce energy.
The difference between normal and cancer cell energy utilisation
Normal cells fluctuate between using sugars or fatty acids depending on blood sugar levels, degree of oxygenation and rate of proliferation required. Usually cells slow glycolysis in the presence of oxygen and low blood sugars, and favour oxidative phosphorylation (OXPHOS). Likewise, when blood sugars drop, they switch to fatty acid metabolism, which by-passes glycolysis. In normal conditions, the cell metabolism consumes energy, of which 70% is supplied by OXPHOS. In hypoxia, however, glycolysis becomes enhanced to compensate for the weakened function of OXPHOS. Glycolysis and OXPHOS essentially cooperate to maintain the cellular energetic balance. Cancer cells appear to lose this regulation and favour glycolysis, despite falling blood sugars. This aerobic glycolysis was first observed by Otto Warburg in the 1920s and was initially thought to be an Achilles heel for cancer cells and a target for drugs and nutritional strategies such as the ketogenic diet. This view, however, has been challenged by recent investigations, which found that the function of mitochondrial OXPHOS in many cancers is intact and cancer cells can switch from one to another with ease depending on the level of oxygen and other cellular conditions.
.